An unbiased approach to antibody drug discovery
Conventional drug discovery often focuses on a narrow set of mechanisms or therapeutic hypotheses.
At Compass, we take a broad and unbiased approach aimed at identifying synergistic and potentially unexpected combinations in a truly empirical way.
Our workflow is based on a series of proprietary and fully integrated technologies that allow us to rapidly generate and screen highly diverse sets of monoclonal and multispecific antibodies.
The end result is differentiated therapeutic candidates fully optimized for format, epitope, and affinity.
The Compass platform
Common light chain-focused antibody discovery
Compass has developed proprietary in vitro and in vivo platforms for the generation of highly diverse antibody panels based on a single light chain sequence. More than 40 immune targets have been drugged with these systems delivering thousands of epitopically and functionally varied leads that can be screened for combination synergy as cocktails or bispecifics.
Human display for antibody tuning
Our next-generation mammalian display platform filters for well-behaved antibody clones and allows us to fine-tune specificity, affinity, and cross-reactivity. With our combined antibody discovery workflow, we can go from an antigen to purified panels of optimized monoclonal antibodies produced in mammalian cells in fewer than 8 weeks.
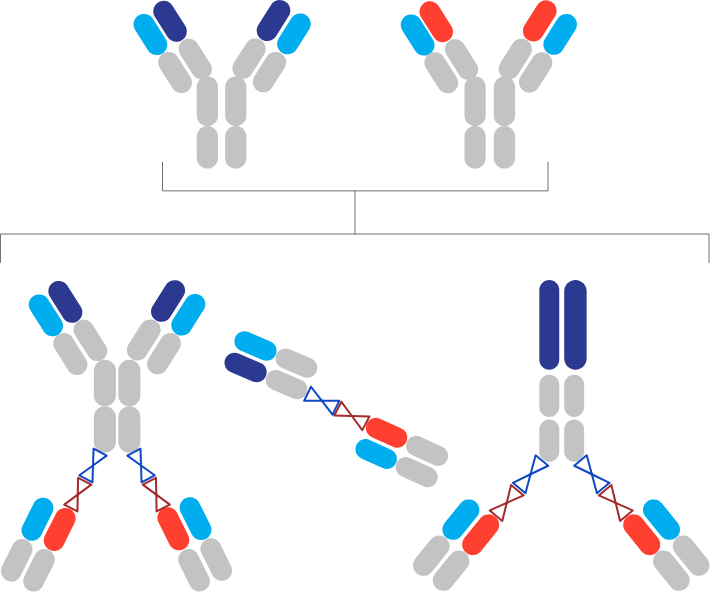
StitchMabs™
Our proprietary, high-throughput multispecific antibody screening platform, StitchMabs™ , allows us to covalently link two or more monoclonal antibodies, antibody fragments, or other biologics into a variety of customized bi- and multispecific formats in a matter of minutes. With StitchMabs™, we can screen bispecific concepts in large, matrix experiments to identify novel and potentially unexpected synergistic combinations.
Highly modular and manufacturable bispecific candidates
Functional insights from the StitchMabs™ platform can be rapidly converted into therapeutic leads by combining the modular common light chain components from our internal discovery. These bispecifics behave like monoclonal antibodies in terms of expression, stability, and pharmacokinetics with diverse formats and valencies tuned to the biological application.
Benefits of the Compass platform:

Speed
We can covalently link antibodies or other biologics into customized formats in a matter of minutes
Modularity
Well-defined components let us tune the valency, geometry, affinity and functionality to attain a precise outcome
Manufacturability
Our multispecifics expression and drug-like properties are comparable to monoclonal antibodies
Through these workflows, thousands of potential monoclonal and multispecific drug candidates have been generated and functionally screened, leading to the identification of differentiated therapeutic candidates.
Our pipeline is focused on therapies that bridge the innate and adaptive immune system and includes novel therapies aimed at either activating or releasing immune suppression of T cells, NK cells, and macrophages.